
The Next Generation Marek’s Disease Vaccine
Providing the right balance between safeguarding bird health, efficacy and performance

What is the importance of Marek’s disease?
Marek’s disease (MD) is a common neoplastic (tumour causing) and immunosuppressive disease affecting poultry populations worldwide. It is caused by the Marek’s disease virus (MDV), which is a ubiquitous alphaherpesvirus. It is costly to the industry and causes unpredictable outbreaks.
Marek’s disease infection occurs mainly in chickens and the virus is known to be endemic in all poultry producing countries around the world. The nature, pattern and presentation of Marek’s disease outbreaks are often complex and unpredictable. The ubiquitous nature of the Marek’s disease virus within the poultry house means that early vaccination in the hatchery plays an essential part in the control of the disease.
The virus is found in dander, litter and feathers, all of which can remain infectious for four to eight months at room temperature. The disease is passed, horizontally, from bird to bird and infection spreads quickly through the flock.
While some birds exhibit clinical signs associated with infection, subclinical Marek’s disease can have a big impact on productivity. Although birds may not show overt clinical signs, the infection can result in decreased egg production and immunosuppression, making the bird more susceptible to other infections and diseases and, in the case of broilers, lead to an increased carcass condemnation rate at slaughter.
Due to clinical and subclinical disease causing decreased performance, Marek’s disease costs the industry $1-2 billion annually¹.
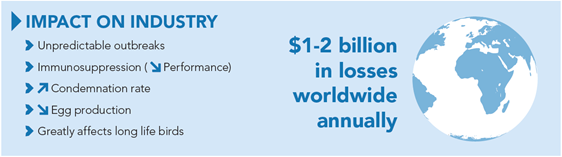
I already vaccinate against MD, why do I need a different vaccine?
MD maintains a constant infection pressure on farms, therefore keeping this disease under control through vaccination is critical². During the past twenty years, several studies have shown the emergence of increasingly virulent strains³. This necessitated the development of a new vaccine.
The clinical picture of MD has changed; most strikingly, the sporadic and chronic nature of the disease has given way to an extremely aggressive, acute disease and protection with previously protective vaccines and immunisation regimens can fail⁴.
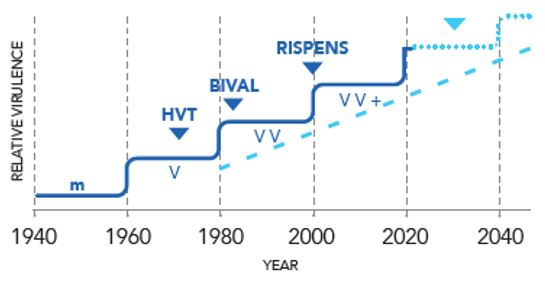
Two generations of Marek’s disease vaccines have shown reduced efficacy over the last half – century due to evolution of the virus⁵.
What is PREVEXXION and why is it different?
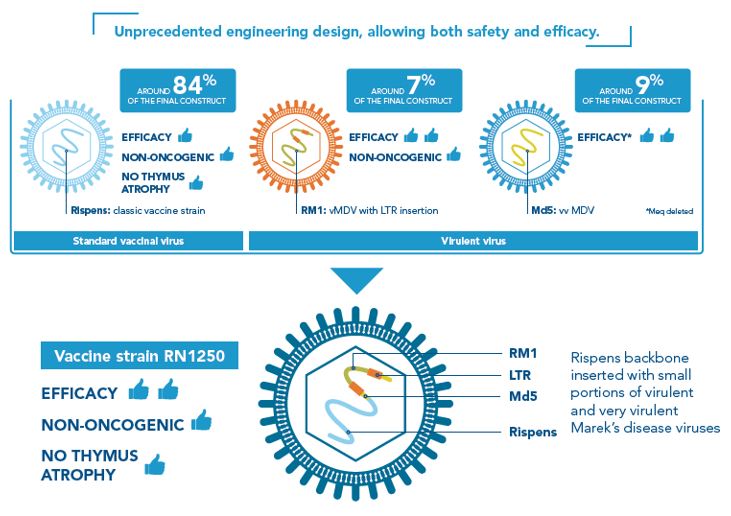
The RN1250 (or RN) vaccine strain of PREVEXXION® is the result of an innovative vaccine engineering design, taking the best traits from three different existing strains of Marek’s disease virus (MDV) and combines them for exceptional protection and efficacy. Thereby creating the first hybrid, next generation, vaccine against Marek’s disease.
Prevexxion® RN protects flocks against very virulent MD challenges6 and does not compromise the productivity of the bird post vaccination³.
PREVEXXION® RN is genetically different from any other existing MD vaccines including CVI988 (Rispens) strains. The vaccine RN1250 strain consists of a backbone of CV1988 Rispens strain (current Serotype-1 MD vaccines use this strain), combined with small portions of virulent (RM1) and very virulent (MD5) MDV strains. In addition to this, Prevexxion® RN has been attenuated by inserting a naturally occurring piece of genetic material called an LTR (long terminal repeat).
Attenuation is when a vaccine virus is altered, so it doesn’t cause disease or harm the host, but can still trigger immunity. Up until the development of Prevexxion® RN, Serotype-1 Marek’s disease vaccines have been attenuated in a way that successfully reduces the ability of the vaccine virus to cause disease but by doing this also reduces the strength of the immune response provided by the vaccine. Prevexxion® RN is attenuated in a new and innovative way by the method of LTR insertion, meaning the vaccine virus is able to stimulate a strong and protective immune response without compromising bird health³.
Producing a vaccine that is effective but also safeguards bird health, is a very difficult balancing act. The innovative vaccine virus construct of Prevexxion® RN achieves this balance, so birds are protected against very virulent MD challenges while maintaining birds’ health.
What are the features of Prevexxion® RN in a nutshell?
There are many features of Prevexxion® RN that makes it convenient to use.
- Prevexxion® RN
- Monovalent vaccine
- Single dose hatchery administration
- Rapid onset of immunity from five days of age
- Application as convenient as for classic MDV vaccines
- Licensed for use in day-old broilers, layers and breeders
- Next generation Marek’s disease vaccine strain protecting against very virulent MDV
- Available in ampoules of 1,000 and 2,000 doses, frozen in liquid nitrogen
- Licensed to mix with Vaxxitek® HVT + IBD
- Also available as RN+HVT+IBD (Prevexxion® RN + HVT +IBD) combined in one ampoule, giving protection against very virulent Marek’s disease and infectious bursal disease (Gumboro), in one single injection
How would I incorporate Prevexxion® RN into my vaccination regime?
Prevexxion® RN is a Marek’s disease vaccine that protects against very virulent MD, therefore it will be of most value to longer lived or more valuable birds, such as layers, breeders and longer-lived broilers, or in any birds where there are perceived problems with Marek’s control. Its application is as convenient as any other MD vaccine this means that however you are currently applying your Marek’s disease cover, Prevexxion® RN could be used, in consultation with your veterinarian, in place of your current vaccine.
Not only can Prevexxion® RN be used as a standalone MD vaccine, if you are also a Vaxxitek® HVT+IBD user, you can mix both vaccines and apply them at the same time. For additional convenience Boehringer Ingelheim have also produced a convenient all-in-one RN, HVT and IBD vaccine (Prevexxion® RN +HVT+IBD) giving you the peace of mind that your birds are being protected against two significant diseases (MD and IBD) that threaten the productivity of your flock. As for any discussion about vaccination regimes and appropriate vaccination protocols for your stock, please discuss your options with your poultry veterinarian.
- Morrow C., Fehler F. 2004, Marek’s Disease: An Evolving Problem. p 49–61 in Davison F, Nair V. Elsevier, London, United Kingdom.
- Gimeno I.M,, 2008, Vaccine, 26S, C31-C41
- Witter R.L. & al., 1997, Avian Dis, 41, 407-421
- Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek’s disease virus: from miasma to model. Nature Reviews Microbiology;4(4):283, 2006.
- Gimeno, I. et al. Avian Pathology, 40(6), 573-579, 2011
- Marek’s disease protection demonstrated through field and laboratory experiences throughout the globe. Trial results are available upon request.
Prevexxion® RN contains cell-associated, live recombinant Marek’s disease (MD) virus, serotype 1, strain RN1250: 2.9 to 3.9 log10 PFU*. UK: POM-V. Prevexxion® RN+HVT+IBD contains cell-associated, live recombinant Marek’s disease (MD) virus, serotype 1, strain RN1250: 2.9 to 3.9 log10 PFU* and cell-associated, live recombinant turkey herpesvirus (HVT), expressing the VP2 protein of infectious bursal disease (IBD) virus, strain vHVT013-69: 3.6 to 4.4 log10 PFU*. UK: POM-V. Vaxxitek® HVT + IBD contains live vHVT013-69 recombinant virus, at least 3.6 to 5.0 log10 PFU*. UK: POM-V. Advice should be sought from the prescriber. Further information available in the SPC or from Boehringer Ingelheim Animal Health UK Ltd., RG12 8YS, UK. UK Tel: 01344 746960 (sales) or 01344 746957 (technical), IE Tel: 01 291 3985 (all queries). Email:vetenquiries@boehringer-ingelheim.com. PREVEXXION® & VAXXITEX® are registered trademarks of the Boehringer Ingelheim Group. ©2021 Boehringer Ingelheim Animal Health UK Ltd. All rights reserved. Date of preparation: May 2021. UI-POU-0009-2021. Use Medicines Responsibly.
*PFU: plaque forming units.


